Epileptic seizure detection
Epilepsy is a neurological disorder that affects 1% to 3% of the world’s population. An epileptic seizure is an abnormal hypersynchronous discharge of cortical neuronal populations (Hauser et al., 1996). Due to brain immaturity, neonates are more prone to epileptic seizures (Volpe, 2000). The majority of neonatal seizures occur within the first few days of life, with an incidence between 1.8 and 3.5 per 1000 live births (Mizrahi, 2001). Early-onset epilepsy can severely interfere with both language and social development in developing newborns, who may be impaired in other domains including coordination, cognition, and behavior (Arzimanoglou and Goutières, 1996). Delay in the diagnosis and treatment of untreated prolonged seizures may result in long-term neurological damage (Holmes and Ben-Ari, 2001). Prolonged seizures can also result in impaired neurodevelopment or even death (Celka et al., 2001). Most neonatal seizures are focal and subtle. In newborns, there are also multifocal seizures or seizures, which migrate from one region to another. Furthermore, the morphology of neonatal seizures varies tremendously between neonates and even within seizures (Fig. 1).
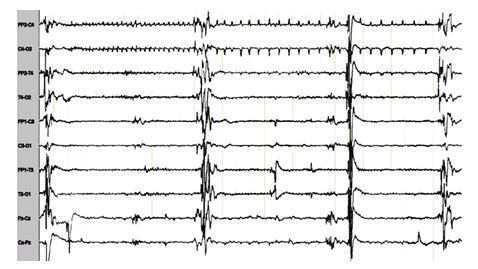
Figure 1. A typical neonatal seizure.
In neonates, as shown in Fig. 2, EEGs are classified into normal, abnormal (discontinuous type A and B and hyperactive rapid activity) and highly abnormal (inactive, paroxysmal, and low voltage plus theta activity) (Lamblin et al., 1999). In this age group, EEG background activities might mimic seizure activities consisting of theta, delta, or alpha-like waves and spike activities (d’Allest and Navelet, 1983). Long-term video-EEG monitoring is an established procedure to capture seizures and paroxysmal non-epileptic events (Lamblin et al., 2004).
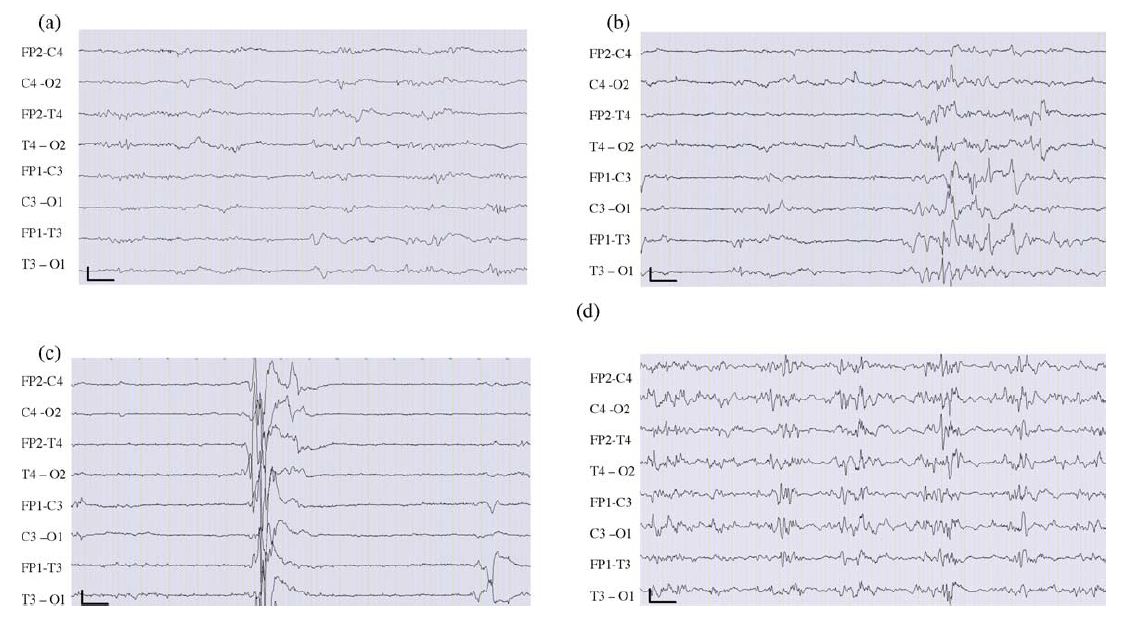
Figure 2. Examples of abnormal background activities in newborn EEG. (a) Type A discontinuous EEG tracing. (b) Type B discontinuous EEG tracing. (c) Paroxysmal tracing. (d) Burst-suppression tracing. Calibration parameters are 1 s and 10 mV.
In traditional methods, EEG experts visually review entire EEG recordings to identify physiological and pathological EEG events. This method is time-consuming, expensive, and requires specialized knowledge. Modern computer systems have allowed it to automatically analyze EEG data using cutting-edge EEG signal analysis methods. Automated methods in medicine can sometimes replace the human expert in cases when there are no risks to patients. The automatic diagnosis can save a lot of time and effort when used with human experts. The design of seizure detection systems is more challenging in newborns than in adults. The majority of the automatic seizure detection algorithms for adults (Gotman, 1990; Osorio et al. 2002; Wilson et al. 2004) result in too many false positives in neonates, caused by:
- Age-dependent time-varying characteristics of EEG signals in neonates.
- Rhythmic and non-rhythmic artifacts, such as jerky movements in newborns.
- Time-varying changes in the morphology of neonatal seizures with specific electrographic signatures.
- Rapid alterations in sleep-wake cycles.
The majority of adult approaches now in use do not take into account the non-stationary nature of the EEG background activity in neonates. A few seizure detection techniques have been developed in newborns by taking into account differences between the characteristics of seizures and EEG background activities in newborn infants and adults (Gotman et al., 1997a, 1997a; Hassanpour et al., 2004; Faul et al., 2005). Most of them lack the distinctive, age-dependent traits of abnormal and normal EEG.
EEG-based neonatal seizure detection
We developed a multistage EEG seizure detection system (Fig. 3) to identify and classify normal and pathological newborn EEGs as well as seizures in newborns with high sensitivity and selectivity. The system provided information about patterns of brain electrical activity, including type, frequency and laterality of seizures (Aarabi et al, 2006, 2007). To develope this system, we included a wide range of normal and abnormal background EEG - discontinuous type A, discontinuous type B, paroxystic and burst suppression from newborns aged between 39 and 42 weeks (Lamblin et al., 1999).
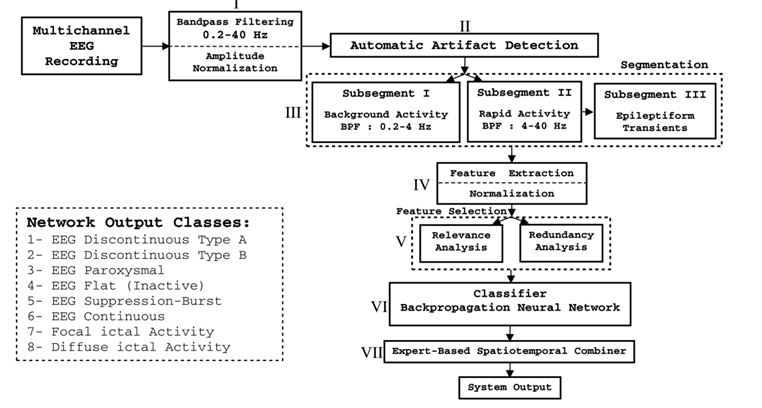
Figure 3. Block diagram of the automatic seizure detection system for newborn infants.
To detect seizures, several characteristic EEG features were extracted from filtered and segmented EEG signals. An optimal feature subset was then selected to best characterize seizure and non-seizure activities. Finally, a classifier was trained to identify optimal boundaries between various classes of signals in the feature space. In this system, (i) activities in newborn EEGs were classified into eight classes (six different types of EEG tracing, ictal and diffuse ictal activities), (ii) an efficient automatic artifact detection procedure was used to identify artifacts, (iii) a nonlinear correlation measure (mutual information) was used to select the best feature subset based on the relevance and redundancy analysis, and (iv) some additional rules were used to reduce false positives. The system was developed and tested on EEG recordings from 10 newborns aged between 39 and 42 weeks. The overall sensitivity, selectivity, and average detection rate of the system were 74%, 70.1%, and 79.7%, respectively. An average false detection of 1.55/h was also achieved by the system with a feature reduction of 80% (Aarabi et al, 2006, 2007).
Seizure detection in intracranial EEG
Intracranial EEG (iEEG) recorded from epileptic patients usually contain epileptiform discharges including spikes, sharp waves, and spike-and-slow wave discharges. Since interictal epileptiform discharges often correlate with the site of seizure onset (Bassel et al., 2003), they show a high degree of similarity with ictal patterns. For this reason, it is difficult for seizure detection systems to assign binary decision labels (‘‘seizure"/ ‘‘non-seizure") to interictal and ictal EEG patterns including interictal and ictal activities. In general, EEG experts use additional criteria based on temporal and spatial contextual information of ictal discharges and interictal epileptiform activities, and other heuristics to visually identify seizures and non-seizure activities. These criteria are usually applied in terms of expert established rules to detect seizures in EEG. These rules are not strict and can be adapted based on the information embedded in interictal and ictal EEG patterns. Therefore, using expert established rules, seizure detection systems would be able to better differentiate ictal discharges from interictal epileptiform activities and to tolerate intersubject variability in ictal and interictal EEG patterns. We presented a novel fuzzy rule-based system for detection of epileptic seizures in iEEG recordings (Fig. 4). Fuzzy inference systems can provide a suitable framework to deal with pattern recognition problems with fuzzy decision boundaries (Bezdek,1981; Zimmermann, 1987). Another advantage of fuzzy rule-based systems is their ability in playing the role of a rule-based interface between features quantified using linguistic information and quantitative measurements (Pedrycz, 1997). In these systems, membership functions are defined for features to provide an estimate of missing or incomplete knowledge. These characteristics give the fuzzy systems a high-level human-like reasoning capability. Our fuzzy rule-based seizure detection system comprised three stages; (1) preprocessing including bandpass filtering, artifact detection, and segmentation, (2) feature extraction, and (3) rule-based decision-making. The last stage mimics experts’ reasoning by appropriately integrating spatial and temporal contextual information of iEEG patterns and rejecting artifacts. The preprocessing stage removes very low-frequency activities and high-frequency noise from segmented iEEG data and automatically detects artifacts. Then, characteristic features are extracted from iEEG segments. Finally, the decision-making block temporally identifies seizure-like activities on each channel independently and then spatially integrates preliminary detections in channels to detect seizures. Simultaneously, based on the characteristics of interictal iEEG, a trained back-propagation neural network estimates thresholds and parameters required for seizure detection.
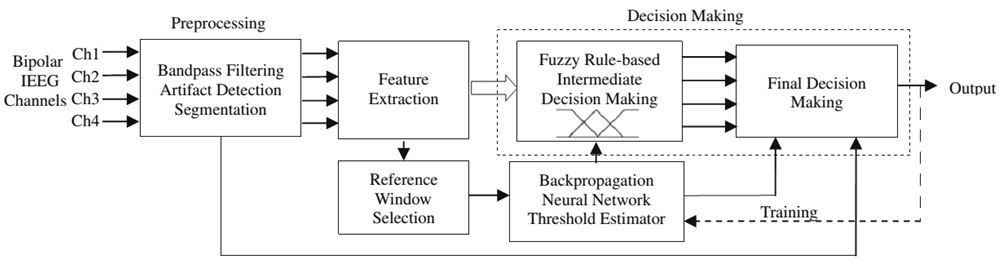
Figure 4. Seizure detection system comprises preprocessing, feature extraction, and decision-making stages. The dashed path was used for training purpose.
A total of 302.7 h of intracranial EEG recordings from 21 patients was used for the evaluation of the system. On average, the system yielded a sensitivity of 98.7%, a false detection rate of 0.27/h, and an average detection latency of 11 s for detection of 78 seizures . The system missed only one short length seizure (Aarabi et al., 2009).
References
Aarabi, A., Wallois, F., Grebe, R., 2006. Automated neonatal seizure detection: a multistage classification system through feature selection based on relevance and redundancy analysis. Clin Neurophysiol 117, 328-340.Aarabi, A., Grebe, R., Wallois, F., 2007. A multistage knowledge-based system for EEG seizure detection in newborn infants. Clin Neurophysiol 118, 2781-2797.
Aarabi, A., Fazel-Rezai, R., Aghakhani, Y., 2009. A fuzzy rule-based system for epileptic seizure detection in intracranial EEG. Clin Neurophysiol 120, 1648-1657.
