
Chercheur récompensé : Jamal EL-ABID
Encadrement : Dr. Vincent CHAGNAULT et Prof. José KOVENSKY

Résumé vulgarisé: Les oligosaccharides sulfatés sont de petites molécules importantes dans divers processus biologiques. Leur efficacité dépend beaucoup du nombre de groupes sulfate qu'ils contiennent et de leur répartition le long de la molécule. Cependant, il est difficile de fabriquer ces molécules, ce qui limite leur utilisation.
C'est pourquoi il est essentiel de rechercher des alternatives. Par exemple, nous avons développé une nouvelle méthode pour créer des molécules similaires aux oligosaccharides sulfatés en remplaçant les groupes sulfate par des groupes sulfonate plus stables en utilisant une méthode plus éco-compatible.
Nous avons ensuite testé ces nouvelles molécules pour voir comment elles interagissent avec certaines protéines et comment elles affectent l'inflammation. Il s'est avéré que l'une de ces molécules est plus efficace pour activer une protéine impliquée dans l'inflammation que son équivalent sulfaté. De plus, une autre molécule s'est révélée être un inhibiteur prometteur d'une enzyme liée à la coagulation sanguine.
En résumé, notre travail vise à créer des molécules similaires aux oligosaccharides sulfatés et à explorer leurs applications potentielles dans le domaine de la biologie et de la santé.
Ferreira Funes, C.; Bouvier, B.; Cézard, C.; Fuentealba, C.; Jamali, A.; Courty, M.; Hadad, C.; Nguyen Van Nhien, A.

Chitin nanocrystals have gained growing interest due to their many excellent properties, however the higher crystallinity of these nano-rod-shaped particles is a major hindering factor in preparation of nanochitosans with low degree of acetylation (DA). Here we studied the effect of the ionic liquid 1-ethyl-3-methylimidazolium acetate (IL) and deep eutectic solvent (choline chloride:lactic acid) (DES) pretreatments of chitin nanocrystals (NCChits) before the deacetylation step using both experimental and theoretical approaches. The results showed that the ionic liquid pretreatment was able to partially disrupt the crystalline structure leading to a lower DA (18.2 %) after two cycles of deacetylation reaction. DES pretreatment, however, was unable to disturb the intra- and intermolecular hydrogen bonds, resulting in a DA of 73.6 % similar to that of unpretreated chitin nanocrystals (73.2 %). SEM images of chitin nanocrystals pretreated with ionic liquid showed that the crystals can rearrange into sheets. Molecular simulations reveal the detailed mechanism of chitin nanocrystal dissociation, in which the combination of a net molecular charge and hydrogen-bonding groups on a single scaffold (as is the case for [C2mim] and [OAc]) plays a paramount role. These results can open the way to afford controlled nanochitosan sheets from chitin nanocrystals.
Vuillemin, M. E.; Waterlot, C.; Verdin, A.; Laclef, S.; Cézard, C.; Lesur, D.; Sarazin, C.; Courcot, D.; Hadad, C.; Husson, E.; Van Nhien, A. N.

This study aims to investigate the ability of an imidazolium biobased Zwitterionic Ionic Liquids (ZILs) in enhancing the phytoavailability of copper from garden (G) and vineyard (V) soils using the model plant ryegrass. Uncontaminated and artificially contaminated CuSO4 soils, unamended and ZIL-amended soil modalities were designed. The copper/ZIL molar ratio (1/4) introduced was rationally established based on molecular modeling and on the maximal copper concentration in artificially contaminated soil. Higher accumulation of copper in the shoots was detected for the uncontaminated and copper contaminated ZIL amended V soils (18.9 and 23.3 mg.kg−1, respectively) contrary to G soils together with a ZIL concentration of around 3% w/w detected by LC-MS analyses. These data evidenced a Cu-accumulation improvement of 38 and 66% compared to non-amended V soils (13.6 and 13.9 mg.kg−1 respectively). ZIL would be mainly present under Cu(II)-ZIL4 complexes in the shoots. The impact on the chemical composition of shoot were also studied. The results show that depending on the soils modalitity, the presence of free copper and/or ZIL led to different chemical compositions in lignin and monomeric sugar contents. In the biorefinery context, performances of enzymatic hydrolysis of shoots were also related to the presence of both ZIL and copper under free or complex forms. Ecotoxicity assessment of the vineyard soil samples indicated that the quantity of copper and ZIL remaining in the soils had no significant toxicity. ZIL amendment in a copper-contaminated soil was demonstrated as being a promising way to promote the valorization of phytoremediation plants.
Ndour, M.; Bonnet, J.-P.; Cavalaglio, S.; Lombard, T.; Courty, M.; Aymard, L.; Przybylski, C.; Bonnet, V.
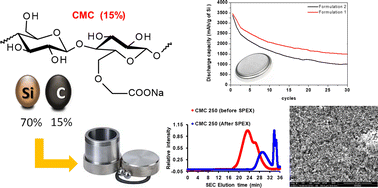
The semi-synthetic polysaccharide carboxymethylcellulose (CMC) is one of the most studied and effective polymer binders for silicon-based anodes in Li-ion batteries. The formulation of the corresponding composite negative electrode with an appropriate mixture of electroactive silicon, a CMC binder and a carbon additive is mandatory to ensure a good electrical conductivity. Blending is commonly realized by a highly energetic ball milling treatment of these three aforementioned components. This type of mixing reduces the size of the obtained particles and can also potentially agglomerate them. Morever, it allows the formation of a nanostructured mixture which is essential for both the silicon activation and to achieve good electrochemical performance. However, such strong treatment can also cause a significant degradation of the polymer chains, as we have recently demonstrated for polyacrylic acid (PAA). In the present work, the structural and chemical effects of this mechanical grinding on three commercial CMCs ranging from 90 to 700 kg mol−1 were investigated. All the polymers were characterized using SEC-MALLS, FTIR spectroscopy, MALDI-TOF mass spectrometry and TGA-MS thermal analysis. In all cases, a huge average molecular weight decrease was noticed, leading to the appearance of a bimodal distribution with low (52–72 kg mol−1) to very low molecular weight populations (1–1.8 kg mol−1). From these results, two formulations of a negative electrode were compared, one with ball milling of the three compounds and another one including only ball milling steps for silicon and carbon. After the correlation of the characteristics of this negative electrode composite with the electrochemical results, it was demonstrated that a high number of functions for supramolecular or covalent linkages are keypoints of the herein anode performance. Low molecular weight CMC derivatives (about 64 kg mol−1) obtained by ball milling treatment led to higher stability of the electrode.